
WHOLE ORGAN IMAGING
Introducing the Kratoscope
FAST AND EASY EX VIVO 3D ORGAN IMAGING
Kaer Labs innovate and introduce the Kratoscope: an easy and efficient way to imaging an entire organ ex vivo.
Although there are more and more techniques to get 3D images of organs (eg. micro-CT, light sheet), none is as simple as "Kratoscopy": the technique does not require any labeling or contrast agent, and the acquisition of a paraffin embedded or frozen organ is usually obtained in less than 2 hours. The technique can however reveal fluorescence signal - in which case it may be referred to as CFT for Cryo Fluorescence Tomography.
The technique, developed in collaboration with the microscopy and histology platform MicroPICell from Nantes University / SFR François Bonamy, is based on the principle of serial block face imaging: the imaging system Kratoscope acquires an image of the organ in the block of tissue each time a slice is cut by a microtome (FFPE blocks) or a cryostat (frozen tissue), in a fully automated process. By getting a stack of slices at increasing depth, it is then possible to reconstruct a 3D view of the organ.
In addition to a possible fluorescent signal detection, the contrast of the images comes from the difference in autofluorescence of different structures of the organ and highlights anatomical its anatomy. It can provide information not visible on micro CT images, when small structures lack radio contrast.
Applications/organs:
The technique has already been applied to a variety of organs, and for different purposes. It has been shown that it is possible on certain organs to isolate the vessels, which appear with a negative contrast, and then to segment them for the characterization and quantification of the vascular tree: this has been done on livers an kidneys, for example. Hearts reveal their inner chambers and the structures of the valves, whereas the Kratoscope is also being used to look at airways of lungs. Brain, kidneys and intestines have also shown to provide good contrast of their inner structures.
The technique can also be used on organs for larger animals (eg. sheep), marine species like zebrafish, as well as for plants for agronomic or botanical studies.
When used to detect fluorescent signals in frozen tissue, the technique is very interesting for 3D biodistribution of fluorescent molecules or particles within organs. When combined with blood vessel staining, it can reveal the position of such molecules/particles with regards to vessels.
Application pages:
Fluorescence Targeting of Glioblastoma
Advantages:
- Different source of contrast than microCT: structures can be easier to observe
- Virtually no specific preparation required: the technique is easier than light sheet, which requires an initial step of tissue clearing
- Minimal operator time: the acquisition process is automated
- Improved repeatability with the absence of staining/labeling
- Individual tissue slices can be collected for further processing and imaging
-
View and measure in 3D
-
Quantify organ spatial structures
-
Simplify preparations and 3D acquisitions

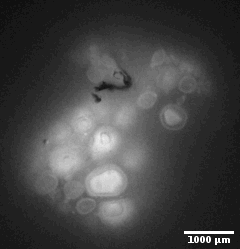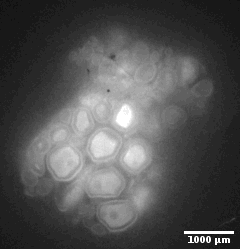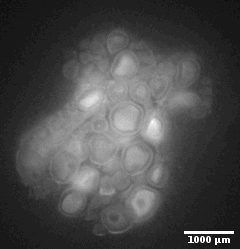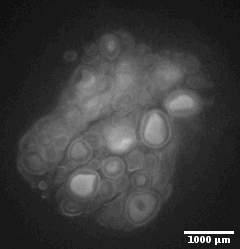
Ewe cervix
Image stack of a cervix from ewe. Slice thickness: 10µm Courtesy of Xavier Druart, INRAE Centre Val de Loire, Nouzilly, France.
Medaka ovary
Image stack of histology slices of a medaka ovary. Slice thickness: 10µm Courtesy Violette Thermes, LGPG, INRAE Rennes, France


Mouse heart
3D reconstruction of a mouse heart. Courtesy MicroPICell, Nantes, France
Mouse lung
Stack of 238 images from a mouse lung, showing lung airways. Slice thickness: 10 µm. Field of view 24 x 24 mm2, zoom position Z0. Courtesy MicroPICell, Nantes, France

Mouse kidney
Stack of serial block face images from a mouse kidney, showing the kidney architecture based on tissue autofluorescence. Slice thickness: 10 µm. Field of view 24 x 24 mm2, zoom position Z0. Courtesy MicroPICell, Nantes, France

Zebrafish
3D reconstruction from whole body zebrafish image stack acquisition. Slice thickness: 10 µm. Field of view 10.7 x 10.7 mm2, zoom position Z1. Courtesy JJ Lareyre, INRAE Laboratory of Fish Physiology and Genomics (LPGP), BIOSIT, Rennes, France
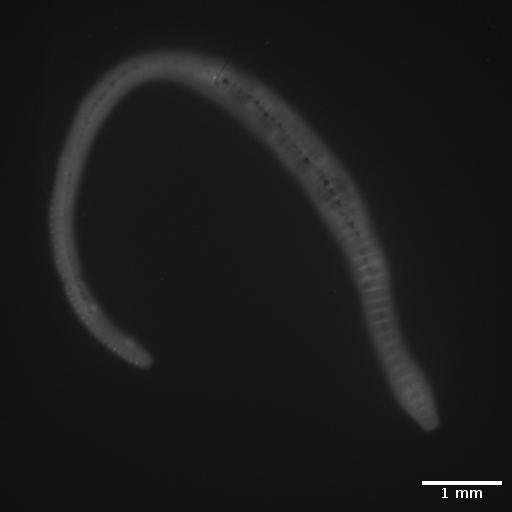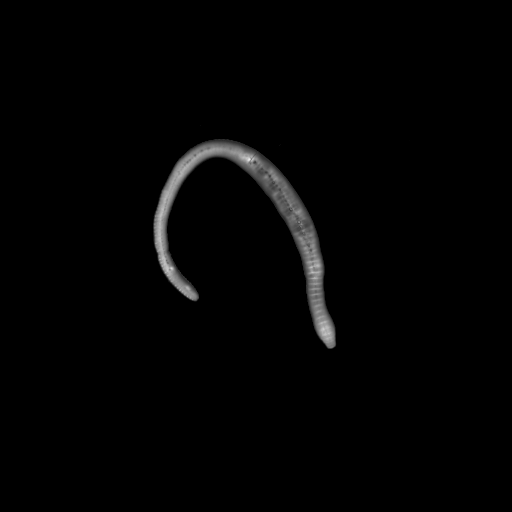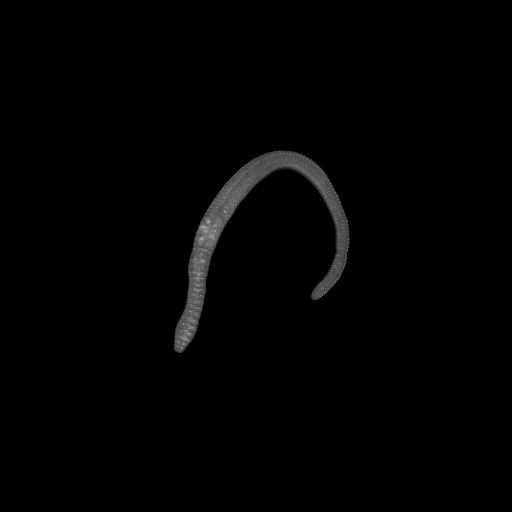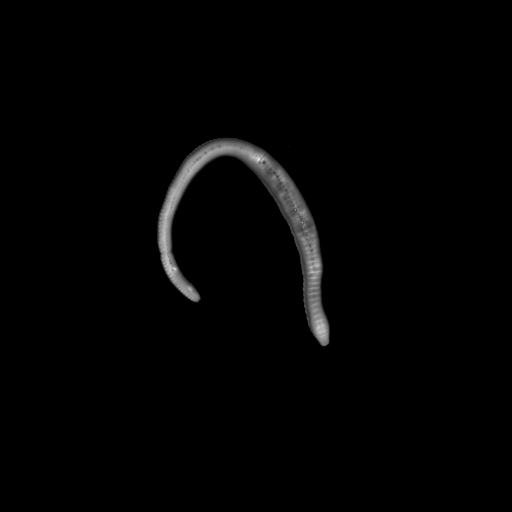
Earthworm
3D view of an earthworm, inside and outside. Slice thickness: 10 µm. Field of view 24 x 24 mm2, zoom position Z0

Zebrafish
3D segmentation and quantitative volumetric analysis of zebrafish ovaries on frozen tissue. Slice thickness: 20 µm. Field of view 10.7 x 10.7 mm2, zoom position Z1. Courtesy JJ Lareyre, INRAE Laboratory of Fish Physiology and Genomics (LPGP), BIOSIT, Rennes, France
Bibliography
The list of publications related to studies performed with the Kratoscope can be found here.
Kratoscopy as a service

In collaboration with the microscopy and histology platform MicroPICell from Nantes University / SFR François Bonamy, Kaer Labs can propose the technique as a service. It is as simple as shipping us your organs embedded in paraffin. Contact us for more information on prices and processes.